Help/FAQ
Browse
You can browse compounds, species, and references lists. Click on the Browse -> Compounds, Browse -> Species, and Browse -> References.Searches
In TabernaDB each metabolite is connected to the producer species of the genus Tabernaemontana.
Compounds
If you know the name of the compound of interest, click on the Search -> Keyword, then just type in the name.
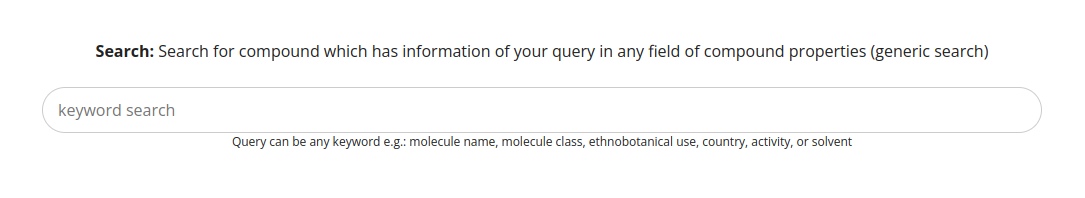
Furthermore, there is the possibility to search for compounds ordered alphabetically. To do so, navigate to Browse -> Compounds.
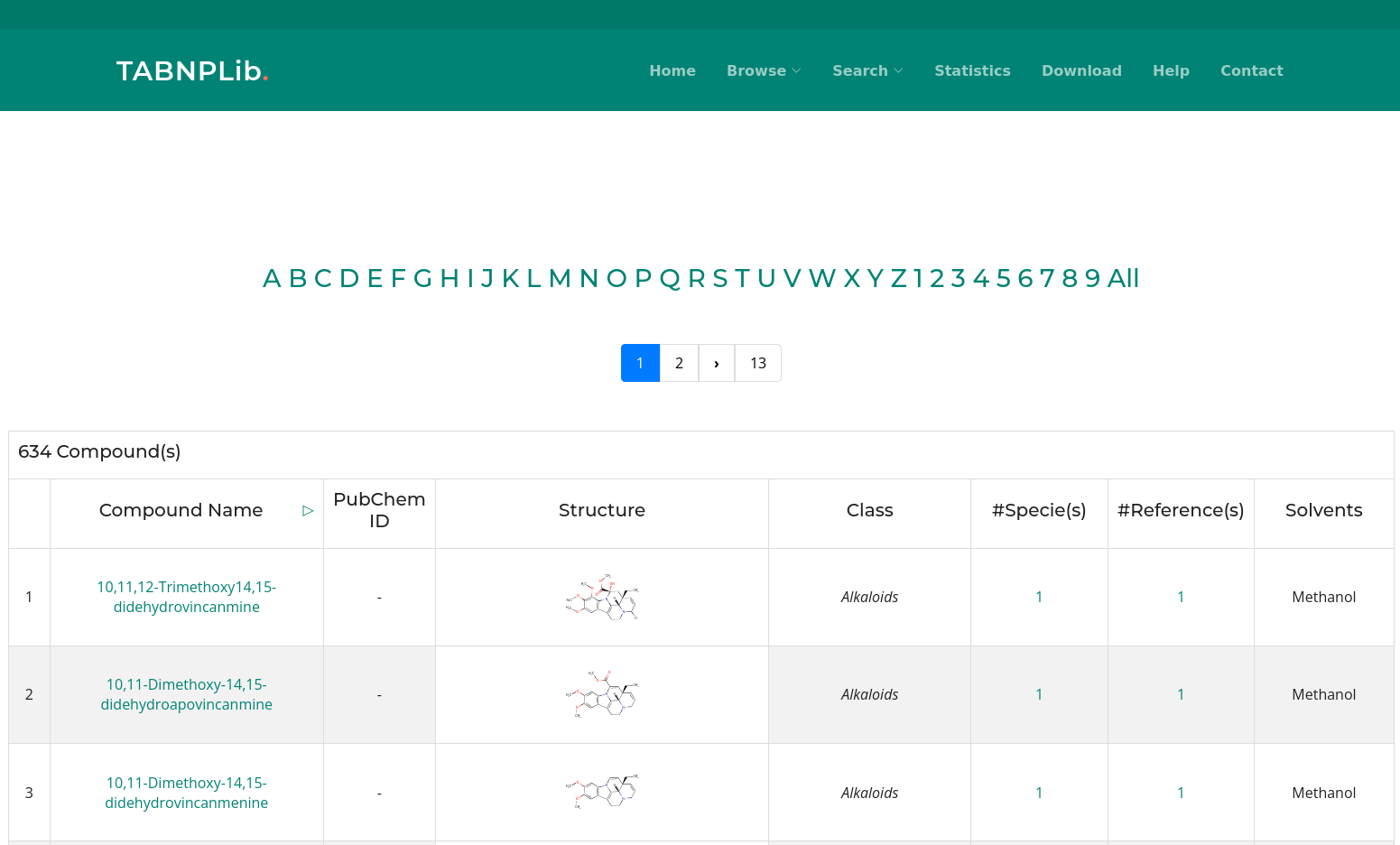
Similarity/Structure Search
Sometimes, the name of a compound is not clearly defined or not known, but the structure of the compound is known. In that case it is possible to draw the structure by using the ChemDoodle structure editor. It is not necessary to match the exact structure; similar structures will also be identified. To do so, navigate to Search -> Similarity/Structure.
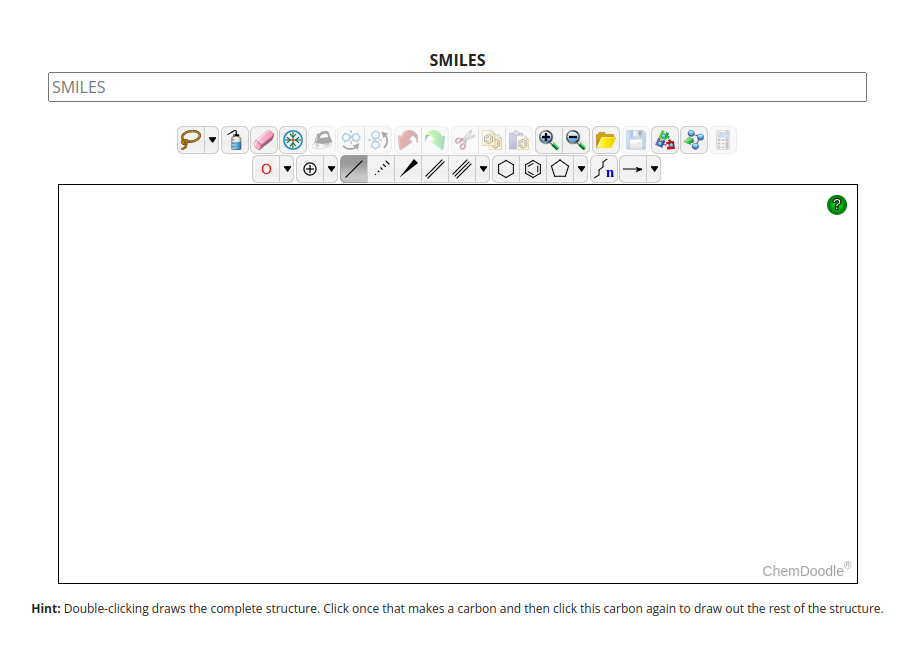
It is possible to change the search parameters, e.g. decreasing the tanimoto coefficient in order to increase the number of retrieved similar compounds. Additionally, you can decide to search for substructures, e.g. you draw a tricyclic structure to get all tricyclic compounds produced by Tabernaemontana species.
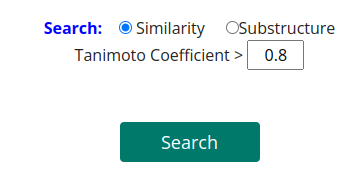
The result window provides a table with identified compounds that are related to TabernaDB species. With a click on the compound name a compound card appears with detailed information.
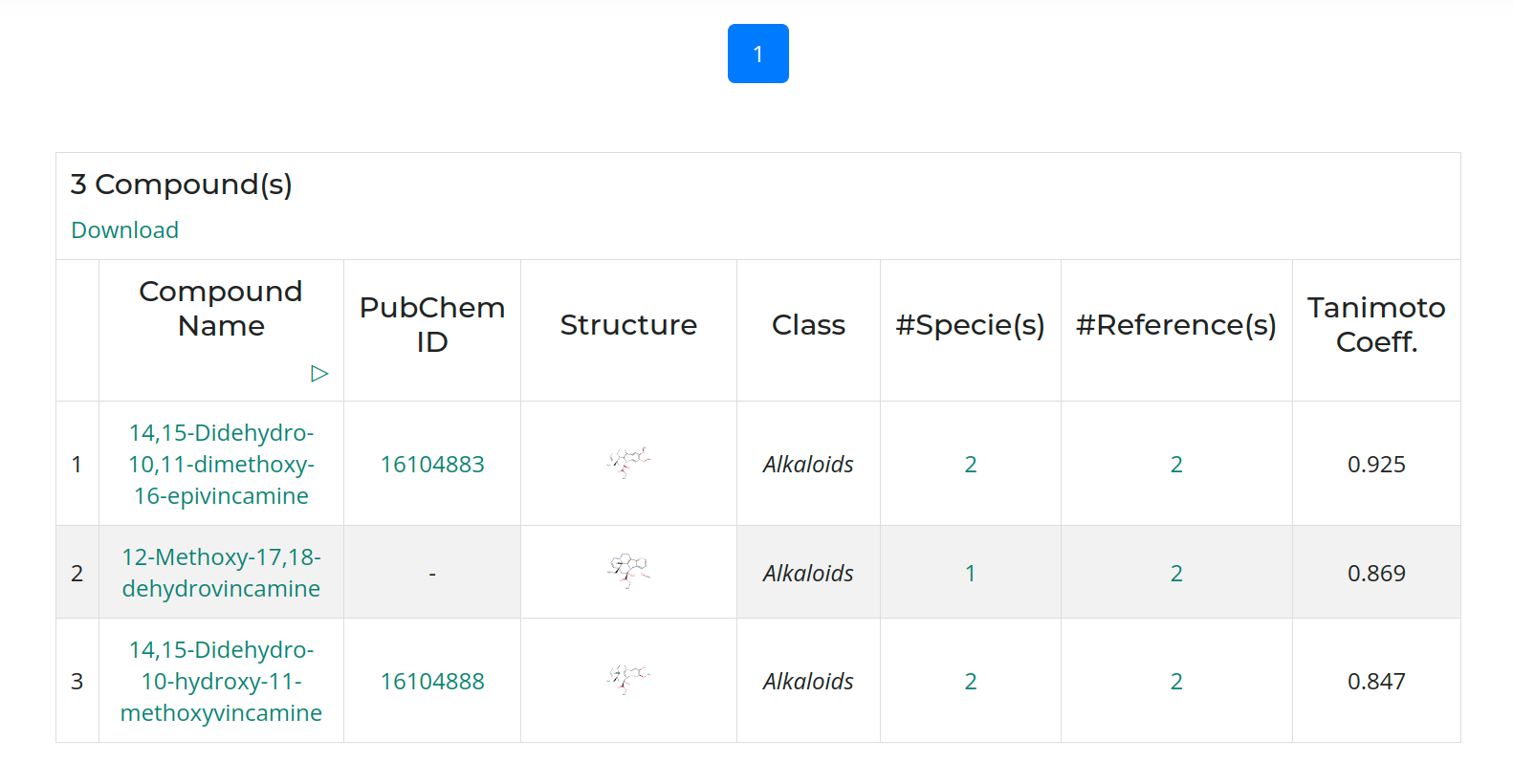
The result table shows resulting species of Tabernaemontana and the number of associated compounds. To get the reference information, click on the number in the column "#References". To get information for the compounds produced by this species, click on the number in the column "#Compounds Associated".
NMR/MS Data
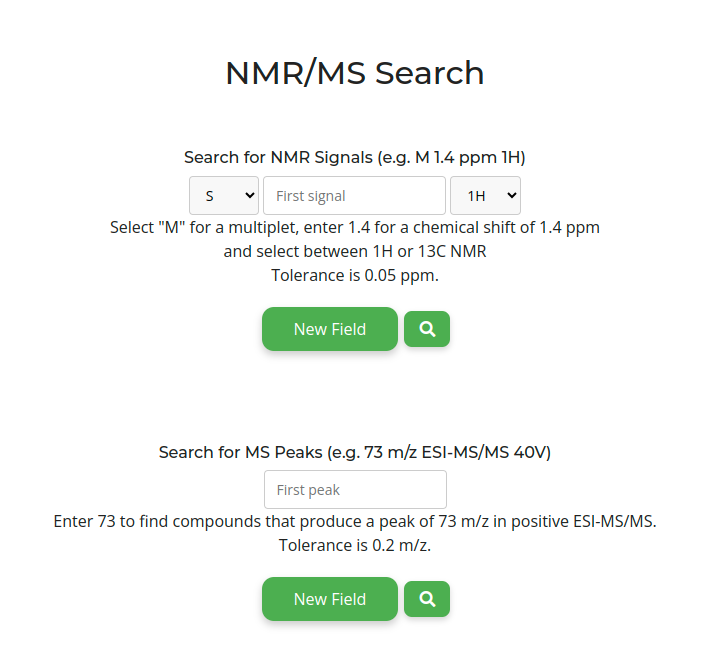
TabernaDB provides thousands of predicted NMR and MS spectra. Search -> Compounds (NMR/MS Data). You can specify multiplicity, chemical shift, and whether you are looking for 1H or 13C results for any number of signals. The multiplicity can be specified to a certain point (S = singlet; D = doublet; T = triplet; D.T = doublet of a triplet; T.T = triplet of a triplet); any other multiplicity can be found by choosing M (multiplet). You can also search for any number of MS peaks by mass-to-charge ratio (tolerance is 0.2 m/z).
2D Similarity Search
Similarity searches in TabernaDB are conducted with Openbabel using 2D Daylight-like fingerprints or substructure searches. Those fingerprints represent structural features of a molecule. Fingerprints are dichotomous [0,1] (bit) arrays. Rather than encoding concrete structural features like structural keys do, there is no assigned meaning to each bit. Fingerprints are generated from the molecule itself using hash algorithms. There are
- patterns for each atom,
- patterns for each atom, nearest neighbors, and respective bonds,
- patterns for each atom group and bonds for paths of up to two, three, ..., and seven bonds in length.
Subsequently, those patterns are generally encoded in four to five bits and added to the fingerprint. If patterns are substructures of another structure, all bits set in the substructure pattern's fingerprint will be set in the other's fingerprint. Boolean operations on those fingerprints provide an effective way for similarity calculations and substructure searches. Stereochemistry as well as atoms not connected to the "main" molecule are not considered in the kind of search TabernaDB perfoms.
TabernaDB uses the tanimoto coefficent for similarity calculations:
tcA,B=NA,B/(NA+NB-NA,B), with
- NA number of bits set "on" in fingerprint of molecule A
- NB number of bits set "on" in fingerprint of molecule B
- NA,B number of bits set "on" in fingerprint of both molecules (A and B).
Features
The next section outlines the features detailed in TabernaDB..
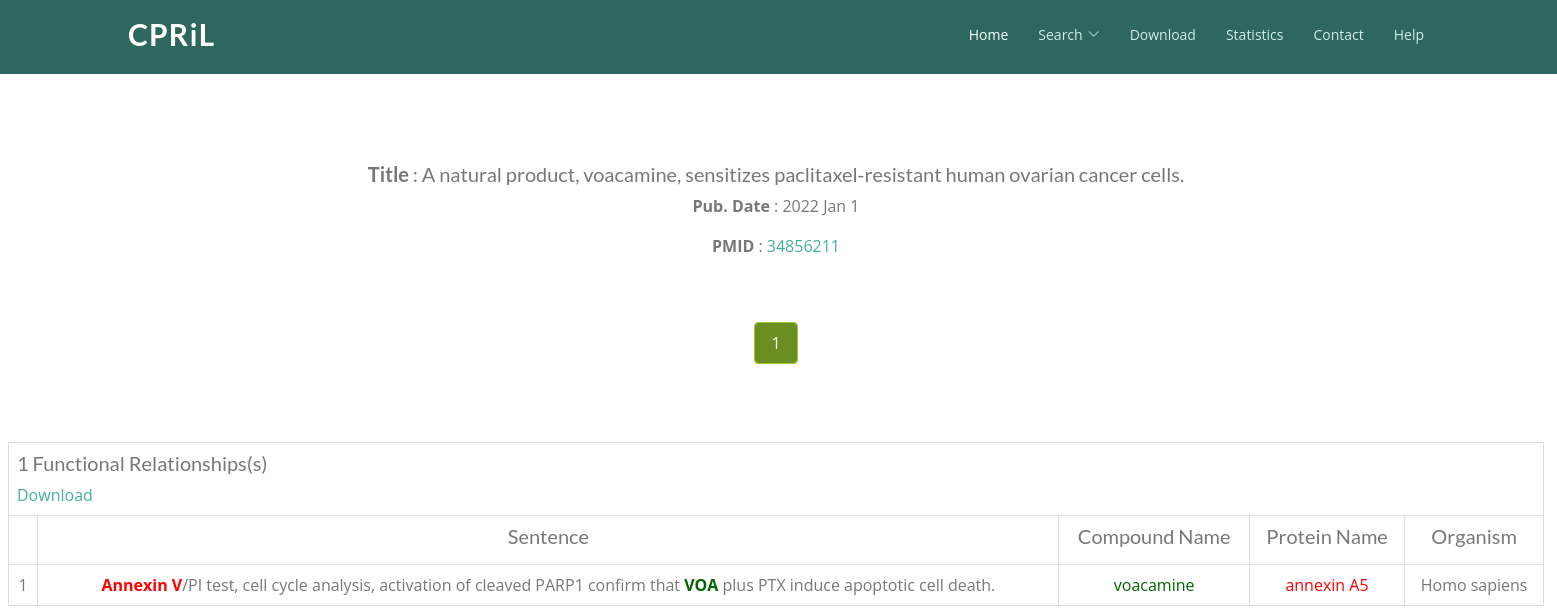
CPRiL (compound-protein relationship)
Click on External Databases -> CPRiL (compound-protein relationship) to be redirected to the corresponding compound card page in CPRiL. On the first page, all functionally related proteins are listed. Click on the Protein Name you are interested in to proceed to the second page.
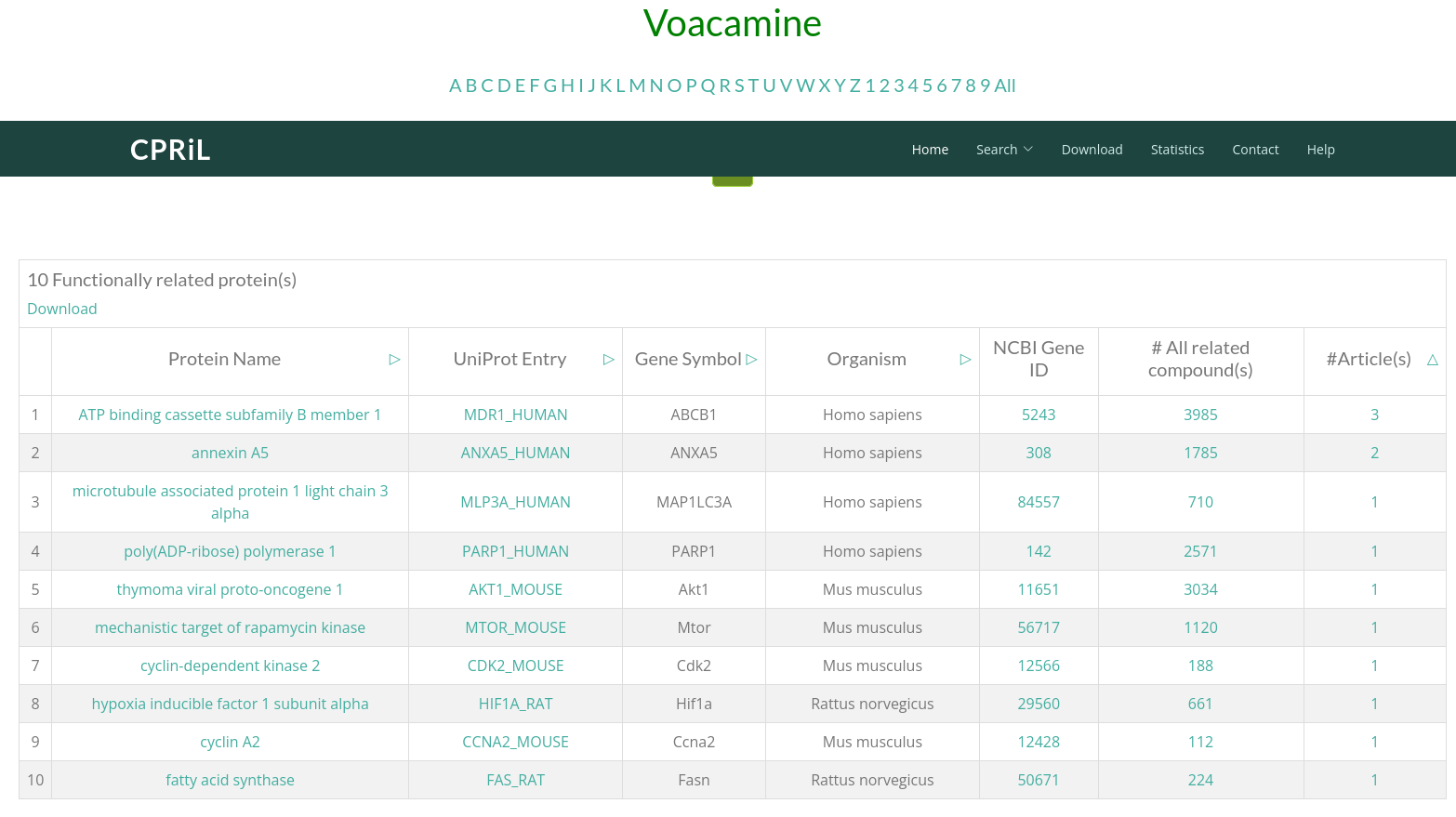
On the second page, you will find all literature documenting the relationship between this protein and the compound. Click on Total Relationships to be redirected to the third page.
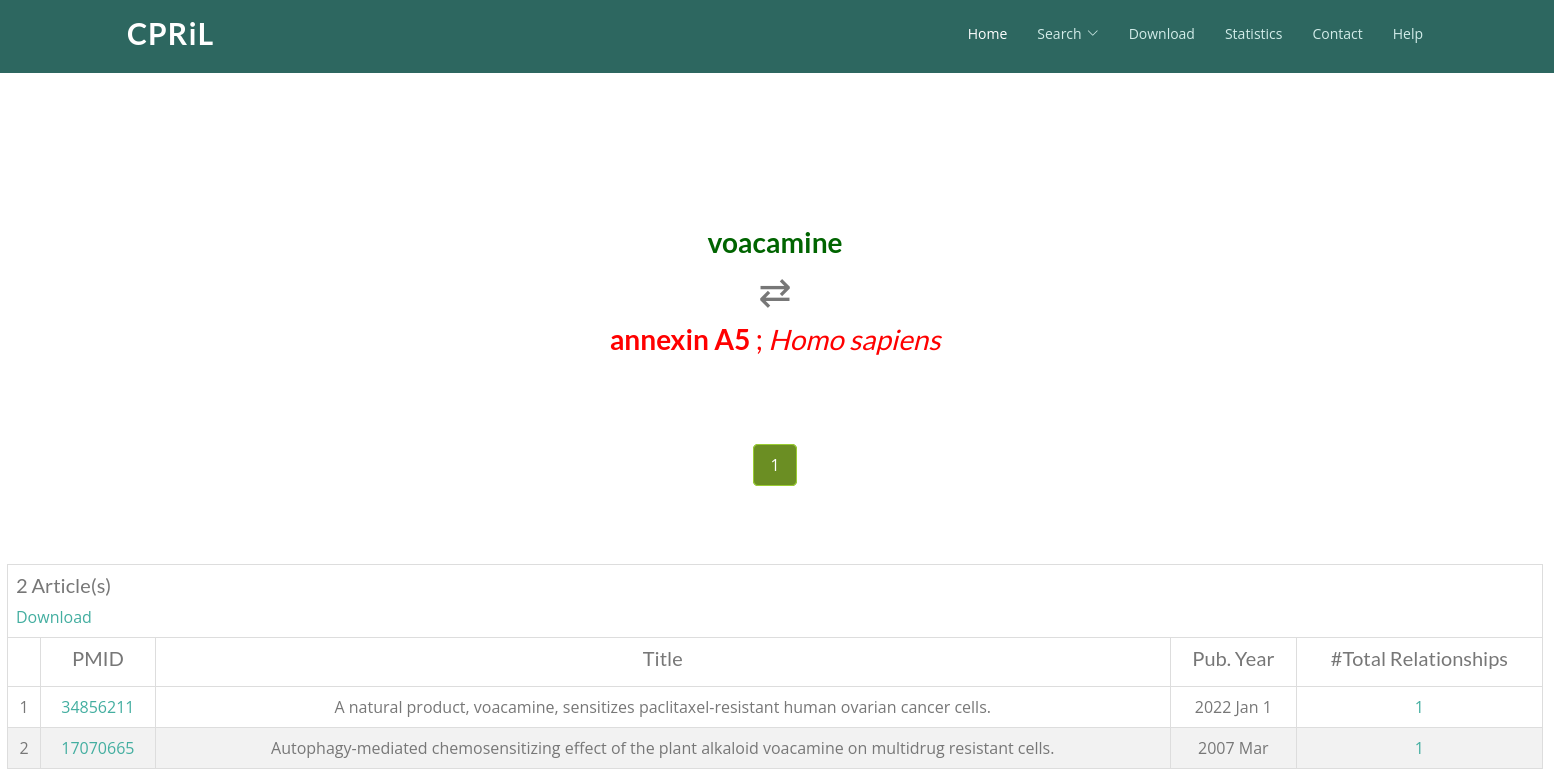
On the third page, you will find the exact sentences from this paper that describe the relationship between the protein and the compound.
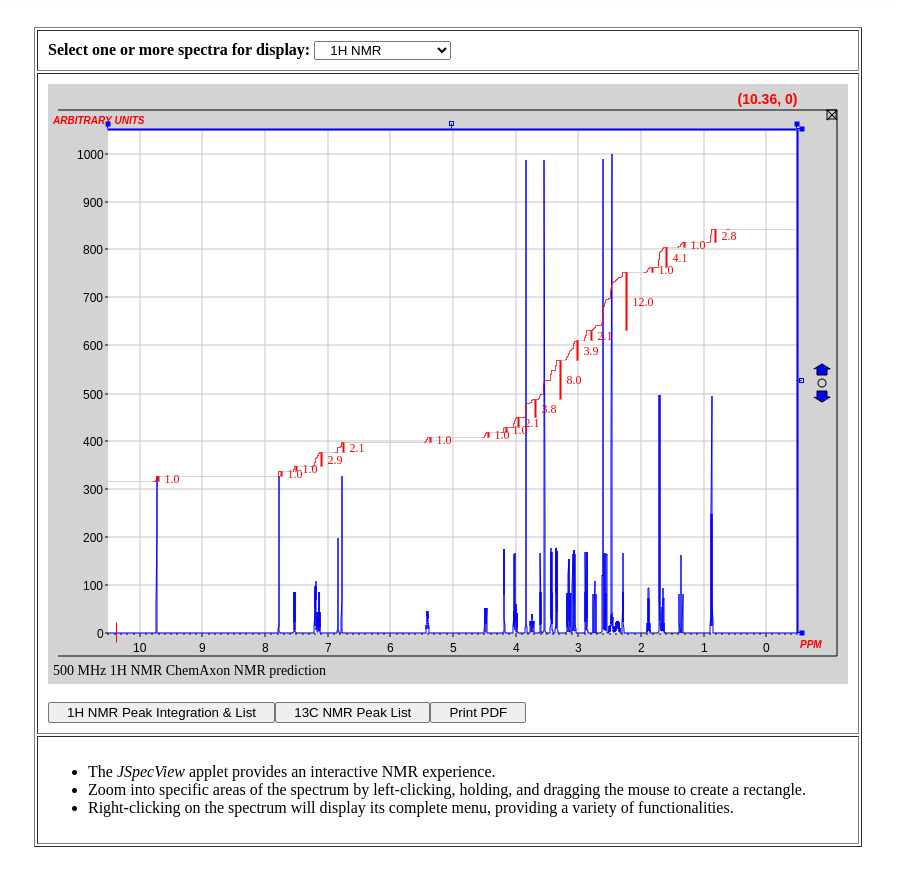
Interactive NMR Spectra
NMR spectra were predicted with the cxcalc command-line tool and the interactive plots were generated with JSpecView. The user can switch between 1H, 13C, or stacked 1H/13C NMR plots. Individual peak lists can be displayed after clicking on a given button below the spectrum.
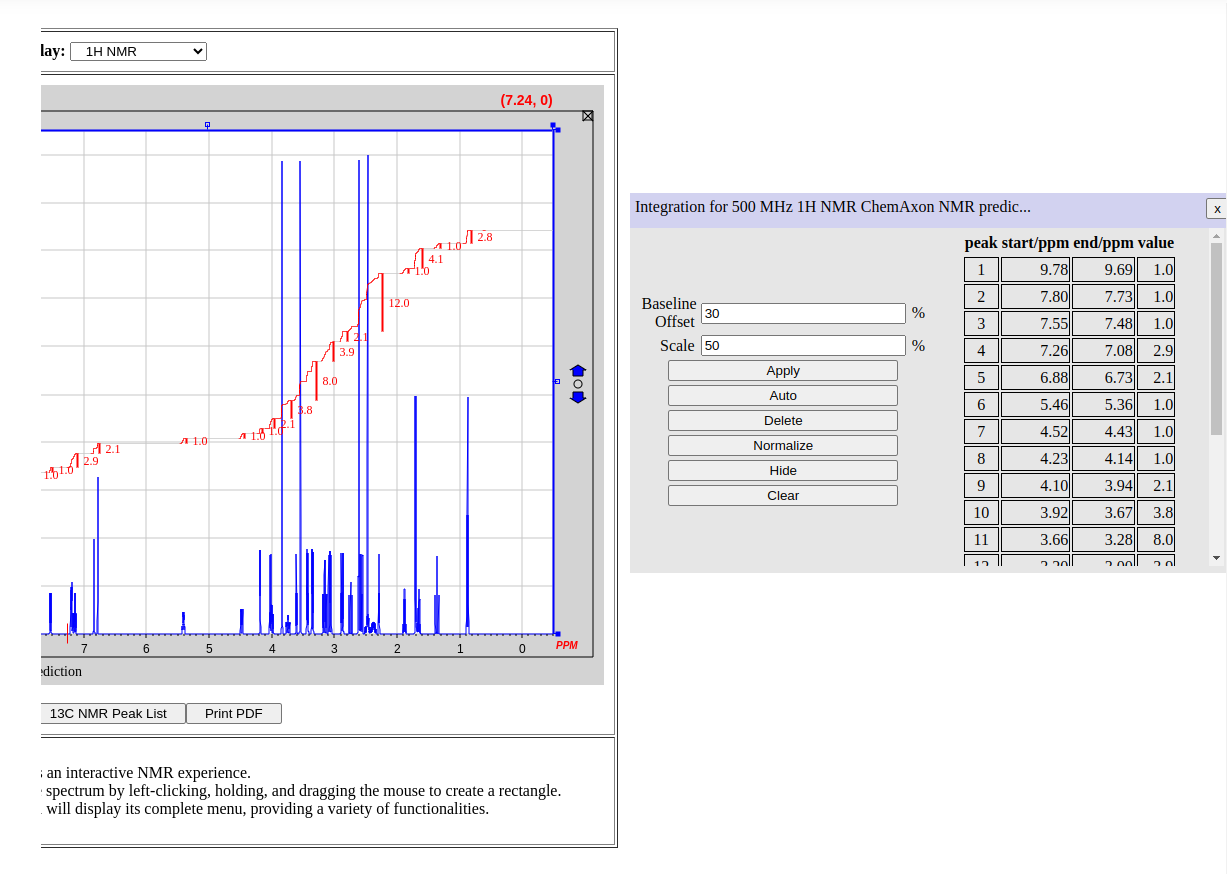
Below is a display of the 1H NMR peak list with corresponding integration values (i.e. number of protons per signal).
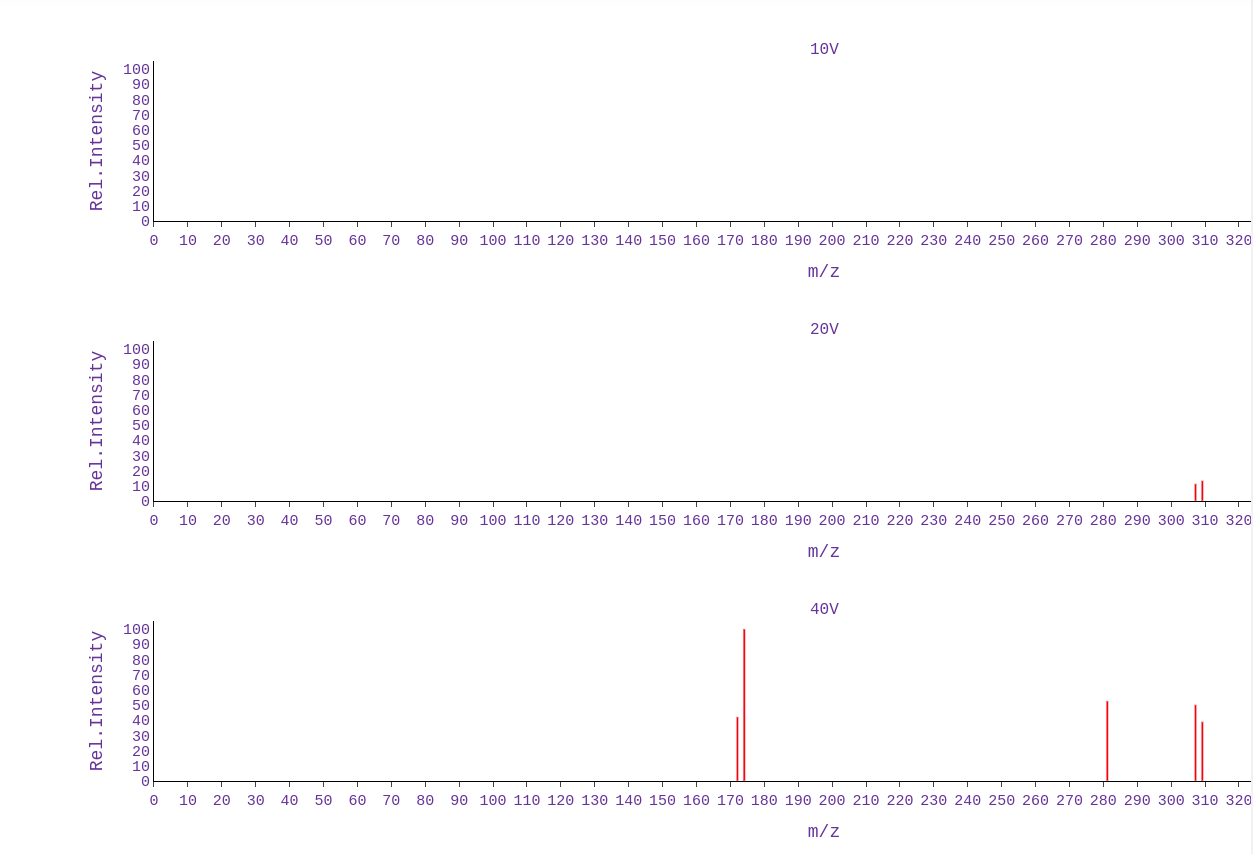
Interactive MS Spectra
MS fragmentation patterns were predicted using CMF-ID, and the interactive plots were generated with plotly.js and displayed in stacked format for 10V, 20V, and 40V. Hovering over a peak will display the predicted fragment structure along with the corresponding m/z value.
FAQs
What is TabernaDB?
TabernaDB is a database that stores information of natural products isolated from a plant of genus Tabernaemontana. These metabolites display various interesting biological activities such as antibacterial, antimicrobial, cytotoxic, and antioxidant properties. In addition to the structures of the compounds, we have included information about source species, references, biological role, activities, etc.
Which input formats can be used for structural searches?
By using the ![]() icon for structures in the editor, you can transfer files in mol, html, and rxnfile formats. To import a structure paste file contents (text) in the text area and press open.
icon for structures in the editor, you can transfer files in mol, html, and rxnfile formats. To import a structure paste file contents (text) in the text area and press open.
What does the tanimoto coefficient tell us?
Fingerprints are {0,1}-strings representing structures (i.e. structure features, properties, or paths) and can be used to calculate similarity coefficients between structures represented by fingerprints. Several widely accepted coefficients exist — we went for the tanimoto coefficient. Values are between 0 (complete dissimilarity) to 1 (identity). We recommend the following paper PMID: 20450209 for further reading.
What is a reasonable cut-off value for the tanimoto coefficient?
For a first try, 0.85 (or 85% similarity) is a widely accepted value. If searches yield too many or too few results (use the 'estimate' option on the 'change parameters' page), you can gradually adjust the tanimoto coefficient to obtain an appropriate number of similar compounds.
What is shown if I click on a compound name?
If you click on a compound name, TabernaDB provides information about the compound, such as synonyms, predicted physicochemical properties, related species including available genomes, corresponding references, additional information about similar compounds, compound activity, the biosynthesises routes, available gene clusters, and scaffolds, among others.
Which browsers can be used?
TabernaDB has been tested and works seamlessly on all major modern browsers, including the latest versions of Google Chrome, Mozilla Firefox, Microsoft Edge, and Apple Safari.
How to cite TabernaDB?
Please cite:
TabernaDB: A Comprehensive Database of Tabernaemontana Natural Products.
Doris E. Ekayen, Ammar Qaseem, Smith B. Babiaka, Yue Feng, Conrad V. Simoben, Andrew E. Egbe, George Chuyong, Stefan Günther (manuscript in preparation).